Are we alone in the Universe?
For the first time in human history, we are on the verge of characterising the atmospheres of distant rocky worlds that could resemble our own Earth. We will be able to search these atmospheres for signs of life, which we call biosignatures. Molecular oxygen (O2), which currently makes up 21% of Earth’s atmosphere, is considered a promising biosignature, since here on Earth it is primarily produced by life.
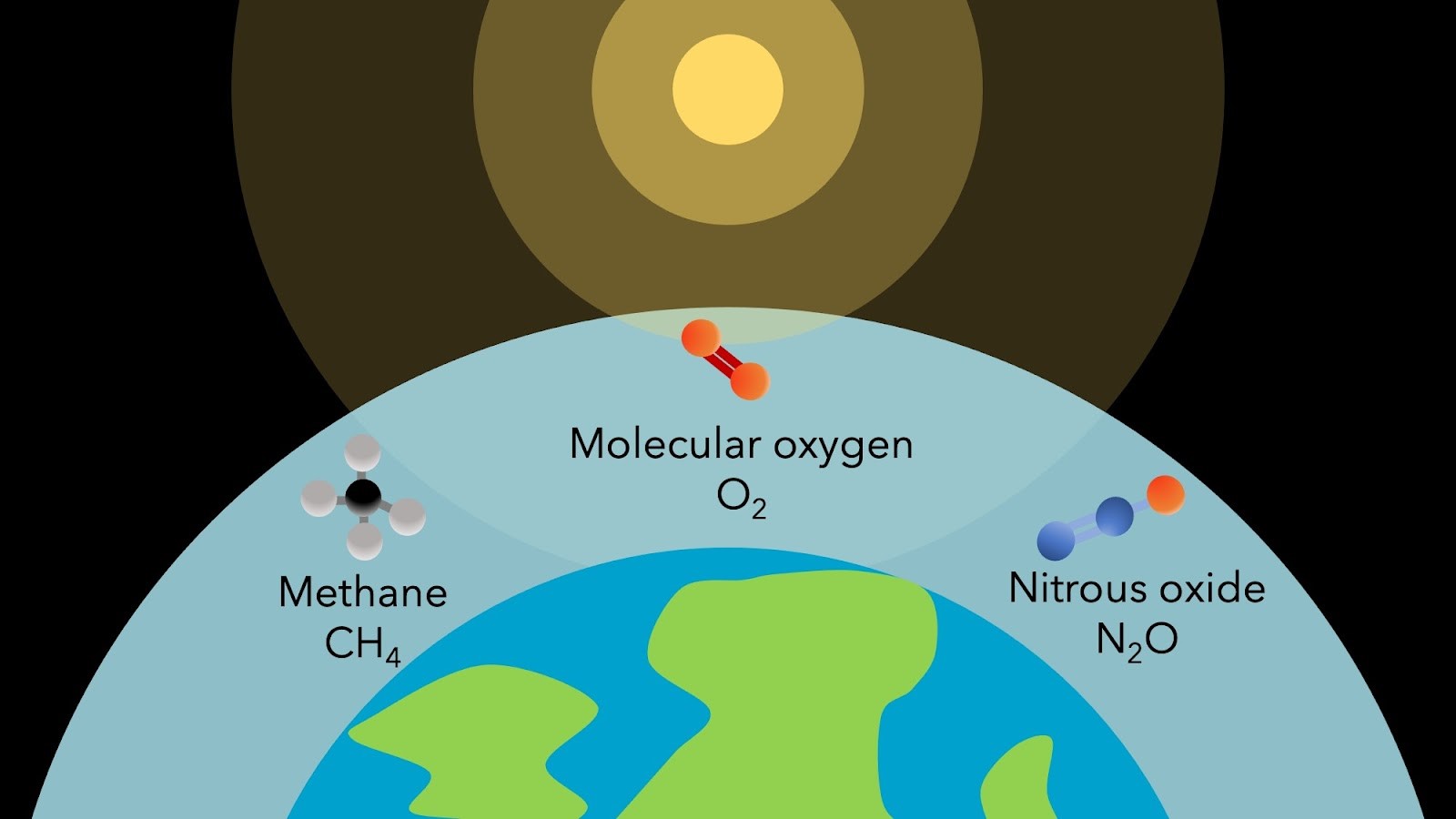
Molecular oxygen (O2), methane (CH4), and nitrous oxide (N2O) are all produced primarily by life on Earth and are considered promising biosignatures. All are strongly impacted by UV radiation from the planet’s host star. Image provided by Thea Kozakis (IAA-CSIC).
Unfortunately, it is often difficult or impossible to detect O2 in certain scenarios. For instance, while the mid-infrared has many opportunities for biosignature detection, O2 detection is not possible. In addition, for the majority of Earth’s history, O2 did not exist in large amounts, even when there was life on the planetary surface. Scientists from the SCITECHSS group are exploring ways to navigate these obstacles to detect life in the Universe using ozone (O3), as a proxy for O2, as ozone is often easier to detect and is a by-product of O2.
“For decades, scientists have been discussing using ozone as a proxy for O2 in the search for life, but our work is the first to explore in detail how to make this idea a reality with future exoplanet observations,” explains our colleague Thea Kozakis.
Learning how to use ozone to infer atmospheric O2
While ozone is not produced by life, it efficiently absorbs harmful ultraviolet (UV) radiation, with Earth’s ozone layer responsible for keeping surface life safe. Not only is ozone detectable at trace amounts as it strongly absorbs light, but it is visible in both the mid-infrared and UV, making it easier to detect than O2. However, O2 and ozone have a highly complicated relationship, strongly influenced by the host star of the planet and the composition and climate of the atmosphere.
To learn how to better use ozone detections as a way to infer O2 content in future observations, Thea Kozakis led a group of scientists who have modelled the chemistry and climate of hypothetical Earth-like atmospheres for planets around a variety of host stars. This study focuses on the impact of nitrous oxide (N2O), also known as “laughing gas”, on our ability to use ozone as a proxy for O2.
Nitrous oxide – or “laughing gas” – can indirectly create or destroy ozone
Nitrous oxide is not only produced by life and considered a biosignature, but creates nitrogen oxides (NOx) via reactions with UV light, which can in turn either create or destroy ozone in different parts of the atmosphere. By modelling the atmospheres of Earth-like planets around a variety of stars, this new study shows how different amounts of nitrous oxide (and therefore NOx) would impact the relationship between ozone and O2.
“Different host stars emit different amounts of UV radiation, which strongly impacts orbiting planets as UV light drives the atmospheric chemistry by breaking apart molecules,” says coauthor João Mendonça, an associate professor at the University of Southampton.
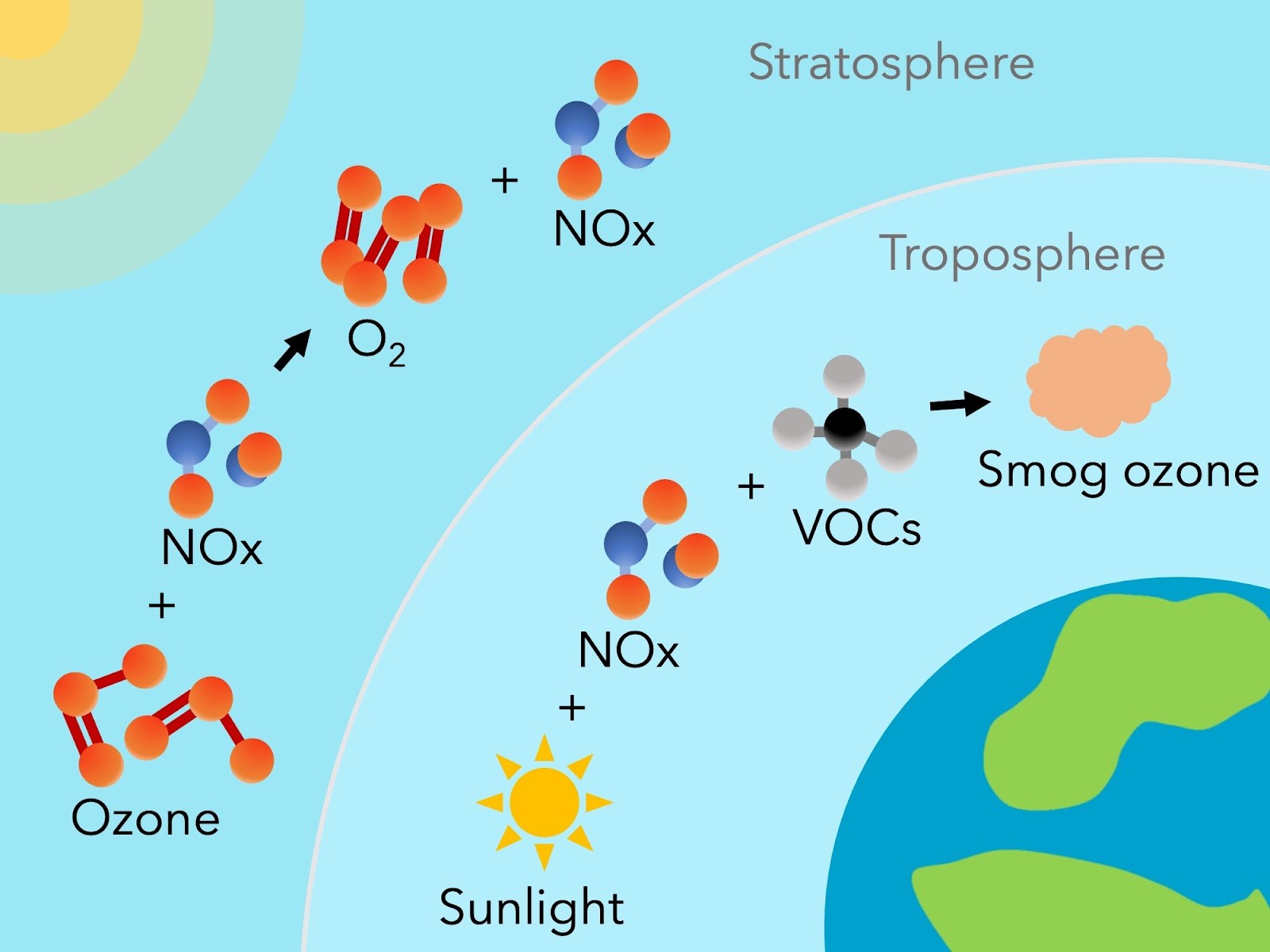
NOx destroys ozone in the stratosphere by converting it back into O2, and creates smog ozone with the help of volatile organic compounds (VOCs) and sunlight in the troposphere. Image provided by Thea Kozakis (IAA-CSIC).
High up in the stratosphere, where the main ozone layer exists on Earth-like planets, NOx efficiently destroys ozone driven by the UV light of Sun-like stars. As ozone is destroyed, more and more harmful UV radiation can reach the ground. In the most extreme case, increasing the nitrous oxide abundance by a factor of 10, the amount of UV reaching the surface increased by a factor of 15 billion. On the other hand, closer to the ground, NOx was shown to be a catalyst for the formation of ozone, especially for planets orbiting cooler stars with less incoming UV radiation. Ozone created close to the planetary surface is referred to as “smog” ozone, and is seen in polluted cities on Earth that have high levels of NOx produced by combustion.
“Anyone who’s lived in a smoggy city knows that ozone near the ground is bad for our health. Although this extra ozone would help shield planetary surfaces from UV radiation, it’s unclear if that could actually encourage surface life, since it’s detrimental to living beings,” explains Thea.
Figuring out the puzzle of planetary atmospheres, one piece at a time
By introducing variations of nitrous oxide to models of Earth-like planets, we are one step closer to understanding how to use ozone as a powerful tool in the search for life in the Universe. The knowledge gained from building up a library of potential Earth-like atmospheres around different star types allows scientists both to strategically plan future observations, as well as design instruments for next-generation telescopes.
“We have a long road ahead of us to fully understand what to look for in biosignature searches, but with every study we can put together a new piece of the puzzle,” Thea reflects.

