We must prepare to search for life in the Universe
With the first light of the Extremely Large Telescope (ELT) on the horizon, as well as multiple next-generation mission concepts in development, we will soon be able to search for life in the Universe with more sensitive tools than ever. However, technological advances alone are not enough for us to accurately identify life on an exoplanet: we must also study what signs of life – known as biosignatures – would look like in different environments. It is important to assess the influence of an exoplanet’s host star on atmospheric biosignatures, as the spectrum of the host star drives both atmospheric chemistry and climate.
The UV spectrum of the host star is particularly important, as UV photons are capable of breaking apart molecules in planetary atmosphere, thus controlling which biosignatures are depleted, and which can build up. Therefore, if we were to put our planet Earth around a cooler star, even if it was at a distance where it could maintain the same surface temperature, the atmosphere could be drastically altered simply by being in a different UV environment.
Ozone is particularly affected by host star UV, and is an important “proxy biosignature”
Atmospheric ozone (O3) is strongly influenced by the spectrum of the host star, as it is created via reactions with UV light. While ozone is not a traditional biosignature since it is not directly produced by life, it is considered an important “proxy biosignature” as it can indicate the presence of molecular oxygen (O2). Molecular oxygen has long been considered a promising sign of life in an exoplanetary atmosphere since on Earth it is produced almost entirely by life, and it comprises roughly 21% of the atmosphere.
However, O2 can be challenging (or impossible) to detect in many scenarios, as it is difficult to detect in small quantities (such as on early Earth), and has no significant spectral features in the mid-IR. Therefore, it has been suggested that ozone, a by-product of O2, could be used as a proxy biosignature since ozone is more easily detectable across a wide range of scenarios. However, because ozone is heavily influenced by the UV spectrum of the host star, it makes it difficult to generalise the O2-ozone relationship, complicating our ability to use ozone to infer the quantity of O2 in exoplanetary atmospheres.
Despite the fact that ozone has been assumed to be a reliable proxy biosignature for O2 for the past several decades, the O2-ozone relationship has only recently been deeply studied in the context of biosignature searches. These studies have been led by SCITECHSS scientist Thea Kozakis, with the latest results examining how methane (CH4) can impact the relationship between O2 and ozone by performing climate
and chemistry modelling of exoplanetary atmospheres.
Methane: a biosignature, and the primary source of water in the stratosphere
While methane itself is a biosignature that is produced biologically by wetlands and livestock, it also indirectly has a significant impact on ozone, and thus the O2-ozone relationship. Notably, CH4 is the primary source of water in the stratosphere, as water itself cannot move from the ground into the upper atmosphere, as it freezes out in the cold trap at the top of the troposphere. Methane, on the other hand, can travel freely from where it is biologically produced on the planetary surface and up into the stratosphere. Through interactions with oxygen and UV light, each CH4 molecule in the stratosphere can be converted into two water molecules.
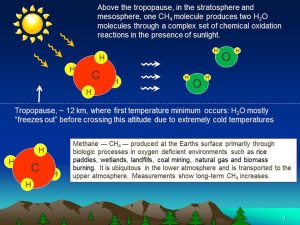
Image: A diagram illustrating the process of stratospheric methane creating water. Methane, unlike water, can travel from the ground into the stratosphere, where reactions with UV can convert one methane molecule into two water molecules. Photo credit: NASA
Water, in turn, has a significant impact on ozone (and therefore the O2-ozone relationship) for two main reasons:
- Water influences the temperature of the stratosphere, and ozone production is particularly sensitive to temperature (favouring cooling temperatures).
- Water creates hydrogen oxides (HOx), which can efficiently destroy ozone through catalytic cycles.
This new study exploring the impact of CH4 on the O2-ozone relationship found that the amount of water created via stratospheric CH4 held the key to determining whether an increase in CH4 would increase ozone formation or suppress it.
Since the conversion of CH4 to water requires UV light, exoplanets orbiting Sun-like stars with high UV fluxes experienced a large increase of stratospheric water when CH4 abundances were increased as well. Although this extra water increased production of hydrogen oxides (which destroy ozone), overall, the ozone abundance increased when compared to scenarios without the added CH4. This increase in ozone despite the higher rates of destruction via hydrogen oxides is due to the indirect impact of CH4 on the stratospheric temperature. Although water efficiently heats the troposphere as a greenhouse gas, in the thin stratosphere, an excess of water causes a cooling effect, as the heat simply escapes to space. This works similarly to the CO2 cooling impacting Earth’s stratosphere, as CO2 increases over time due to human activities.
Although these atmospheres with high CH4 and excess water around Sun-like stars indeed created more hydrogen oxides that destroy ozone, the stratospheric cooling effect increased the rate of ozone production, as it favours lower temperatures. On the other hand, the situation played out differently when increasing CH4 in the atmosphere of a planet orbiting a cooler M dwarf host with a weaker UV spectrum. The lower amount of UV light reaching the exoplanetary atmosphere meant that less CH4 was converted into water, and therefore less water into hydrogen oxides to destroy ozone. However, in these scenarios, adding CH4 to the atmosphere resulted in decreased ozone, in contrast to the scenario around the Sun-like star. This is because, although in both situations CH4 is converted into water in the stratosphere, in the lower UV environment around the M dwarf, not enough water is produced to cause a significant stratospheric cooling effect. Therefore, the change in ozone abundance is completely driven by the increase in hydrogen oxides, destroying ozone, causing an overall decrease in ozone abundance.
Still more work to be done to use ozone as a “proxy biosignature”
The results of this study draw particular attention to how the O2-ozone relationship – and therefore our ability to use ozone as a proxy biosignature for O2 – is further complicated by the impact of CH4 on ozone. Specifically, these studies show how understanding how the UV spectrum of the host star will be of the utmost importance when trying to interpret observations, as it will drive the processes changing the atmosphere. This also highlights the need for atmospheric modelling not just of atmospheric chemistry, but also the temperature of the atmosphere, as many chemical reactions are highly dependent on temperature.
Atmospheric modelling studies like these continue to uncover complex and unexpected atmospheric processes, demonstrating the need for further exploration. Little by little, these investigations help prepare us for the next-generation telescopes that will allow us to search for biosignatures, with each discovery guiding our path.
Link to paper: https://www.aanda.org/articles/aa/pdf/2025/09/aa56015-25.pdf

